
A 75-year-old male with a history of prostate adenocarcinoma (T3b/T4 N1 M1b, Gleason 4+3) on Zoladex® and enzalutamide, presented with visible haematuria.
His prostate specific antigen (PSA) had decreased from 43.6ug/L in December 2022 to 0.02ug/L. He also had a history of hypertension, osteoarthritis, and hypercholesterolemia, with mixed lower urinary tract symptoms (LUTS), managed on tamsulosin. Flexible cystoscopy revealed a large mass on the left lateral bladder wall, with CT imaging confirming a bladder mass and thickening at the bladder dome. Mild pericaecal fat stranding and lymphadenopathy were also noted (Figure 1, 2 and 3). Preoperative blood work showed stable parameters.
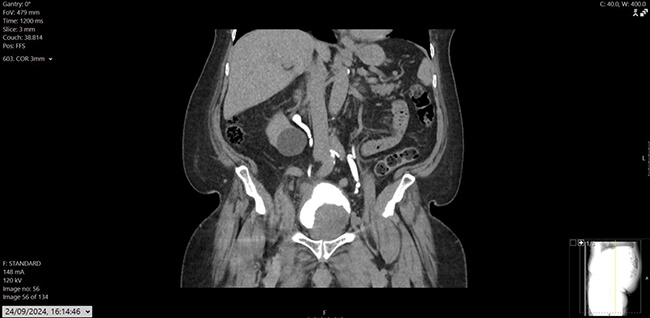
Figure 1: CT Urogram – coronal image showing large intraluminal bladder mass arising from the left lateral wall; another mass like bladder wall thickening noted in the bladder dome extending into the right lateral aspect.
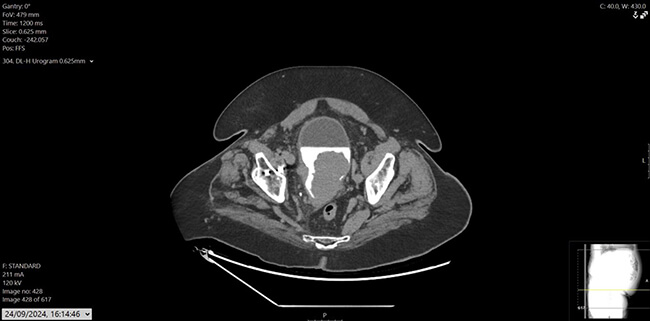
Figure 2: CT urogram, axial.
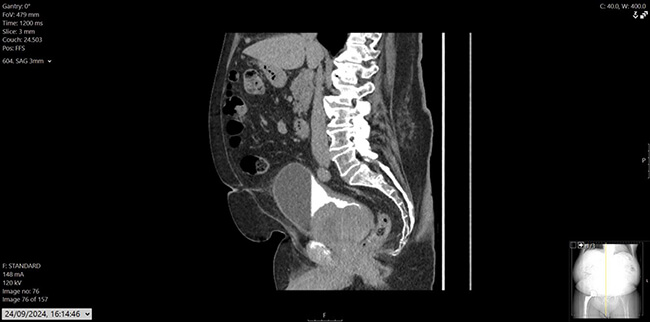
Figure 3: CT urogram, sagittal.
The patient underwent an elective transurethral resection of bladder tumour (TURBT), which was complicated by profuse bleeding, resulting in a postoperative drop in haemoglobin (110g/L). Angiography failed to identify active bleeding, but empirical embolisation of the left prostatic artery was performed. The patient was discharged on postoperative day seven. Histology from the resected tissue showed high-grade carcinoma in situ (CIS) and features of urothelial carcinoma with neuroendocrine differentiation, pT2. Cells were positive for Pan CK (dot-like), synaptophysin, chromogranin and negative for CD45, CD 99 and desmin.
Following surgery, the patient was readmitted two weeks later with clot retention. He also developed new symptoms of malaise, weight loss, dizziness, and imbalance. CT brain and CT chest, abdomen, and pelvis (CAP) scans were performed to rule out metastasis. While no brain metastases were detected, the CT CAP revealed significant progression of the primary tumour, bilateral hydronephrosis, new hepatic metastases, and right-sided pulmonary emboli. Given the rapid disease progression, the patient was palliated and fast-tracked to hospice care for end-of-life management.
Discussion
Neuroendocrine bladder tumours (NEBTs) are a rare and aggressive subset of bladder malignancies characterised by their origin from neuroendocrine cells. These tumours account for less than 1% of all bladder cancers but represent a significant clinical challenge due to their rapid progression, high metastatic potential, and limited treatment options. This literature review summarises current knowledge regarding the epidemiology, pathophysiology, diagnosis, treatment strategies, and prognostic outcomes of NEBTs, with references to key studies.
Epidemiology and clinical presentation
NEBTs predominantly affect older individuals, with a male predominance. The median age of diagnosis is around 60 years, and the majority of cases are diagnosed at an advanced stage [1]. The clinical presentation often mimics that of other bladder cancers, with symptoms including haematuria, dysuria, and urinary frequency. However, due to their aggressive nature, NEBTs are often associated with systemic symptoms, such as weight loss and fatigue, especially in metastatic cases.
Pathophysiology and classification
NEBTs are categorised into small cell neuroendocrine carcinomas (SCNECs), large cell neuroendocrine carcinomas (LCNECs), and well-differentiated neuroendocrine tumours (NETs). SCNECs are the most common subtype and share morphological and molecular features with small cell lung cancer, including TP53 and RB1 mutations [2]. LCNECs and well-differentiated NETs are less common but exhibit distinct histological and molecular characteristics. NEBTs frequently coexist with other histological subtypes, such as urothelial carcinoma, in mixed neuroendocrine-nonneuroendocrine epithelial neoplasms (MiNENs), which are associated with worse outcomes [3].
Diagnosis
The diagnosis of NEBTs relies on a combination of imaging, histopathology, and immunohistochemistry. Imaging modalities such as CT and MRI are used to assess tumour staging and metastases but cannot definitively distinguish NEBTs from other bladder cancers [4]. Histologically, NEBTs exhibit features such as high mitotic activity, necrosis, and neuroendocrine architecture. Immunohistochemical markers, including chromogranin A, synaptophysin, and CD56, are crucial for confirming neuroendocrine differentiation [5].
Treatment approaches
The treatment of NEBTs is challenging due to their rarity and aggressive behaviour. Multimodal approaches are commonly employed, combining surgery, chemotherapy, and radiotherapy. For localised disease, radical cystectomy with pelvic lymph node dissection is the standard surgical treatment [6]. However, systemic chemotherapy is the cornerstone of treatment for metastatic NEBTs, typically involving platinum-based regimens such as cisplatin or carboplatin combined with etoposide. These regimens are adapted from treatment protocols for small cell lung cancer due to the histological similarities [7].
Emerging therapeutic strategies include immunotherapy and targeted therapies. Immune checkpoint inhibitors, such as atezolizumab and pembrolizumab, have shown promise in advanced urothelial carcinomas and are being investigated for NEBTs. Additionally, peptide receptor radionuclide therapy (PRRT) has demonstrated potential in well-differentiated NETs expressing somatostatin receptors, although its application in NEBTs remains experimental [8].
Prognostic outcomes
The prognosis for NEBTs is poor, with a median survival of less than 12 months for metastatic cases. Prognostic factors include tumour stage, histological subtype, and treatment response. SCNECs and LCNECs are associated with worse outcomes compared to well-differentiated NEBTs. The presence of mixed histology, as seen in MiNENs, further worsens prognosis [9,10]. Early detection and aggressive treatment are crucial for improving survival rates.
Conclusion
Neuroendocrine bladder tumours are rare but highly aggressive malignancies with distinct clinical and pathological features. Advances in diagnostic techniques, such as immunohistochemistry and molecular profiling, have improved our understanding of these tumours. Despite limited treatment options, emerging therapies such as immunotherapy and PRRT offer hope for better outcomes. Ongoing research and clinical trials are essential to develop standardised treatment protocols and improve prognostic outcomes for patients with NEBTs.
References
1. Lynch CF, Davila JA, Platz CE. Cancer of the urinary bladder. In: Ries LAG, Young JL, Keel GE, et al (Eds). SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH: 2007.
2. Chang MT, Penson A, Desai NB, et al. Small-cell carcinomas of the bladder and lung are characterized by a convergent but distinct pathogenesis. Clin Cancer Res 2018;24(8):1965–1973.
3. Tran P, Rama Sai P, Prasad C, et al. Mixed neuroendocrine–nonneuroendocrine epithelial neoplasm of muscle invasive bladder cancer: a clinicopathologic case study. J Surg Case Rep 2024;2024(10):rjae612.
4. Pini GM, Uccella S, Corinti M, et al. Primary MiNEN of the urinary bladder: an hitherto undescribed entity composed of large cell neuroendocrine carcinoma and adenocarcinoma with a distinct clinical behaviour: Description of a case and review of the pertinent literature. Virchows Arch 2021;479(1):69–78.
5. Bellizzi AM. Immunohistochemistry in the diagnosis and classification of neuroendocrine neoplasms: what can brown do for you? Hum Pathol 2020;96:8–33.
6. Sarver J, Memo M. Small-cell neuroendocrine tumor of the bladder: A rare disease in a low-risk woman. Uro Case Rep Volume 2021;40:101923.
7. Moretto P, Wood L, Emmenegger U, et al. Management of small cell carcinoma of the bladder: Consensus guidelines from the Canadian Association of Genitourinary Medical Oncologists (CAGMO). Can Urol Assoc J 2013;7(1-2):E44–56.
8. Haider M, Das S, Al-Toubah T, et al. Somatostatin receptor radionuclide therapy in neuroendocrine tumors. Endocr Relat Cancer 2021;28(3):R81–93.
9. Sehgal SS, Wein AJ, Bing Z, et al. Neuroendocrine tumor of the bladder. Rev Urol 2010;12(4):e197–201.
10. Chraïbi M, Barqui M. Small cell neuroendocrine carcinoma of the urinary bladder: rare entity associated to poor prognosis. Int Arch Urol Complic 2019;5:055.







